Physical Sciences/Engineering
Bringing magnetic solutions to breast cancer surgery
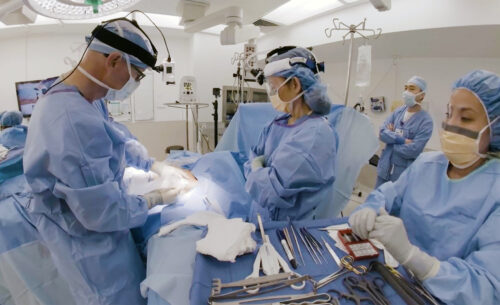
Endomag is a highly innovative UCL med-tech spinout company, which has over 100 employees primarily based out of offices in Cambridge, but also in the US and Germany. The company was created by Professor Quentin Pankhurst and Simon Hattersley from UCL, in collaboration with Professor Audrius Brazdeikis at the University of Houston, and has been strongly supported by UCLB, UCL’s IP commercialisation company, throughout its 15-year translational journey.
Endomag (formerly Endomagnetics) was formed in 2007 to initially capitalise on a simple research idea: to use magnetics to remove the need for radioactivity when staging breast cancer, commonly through a procedure called sentinel lymph node biopsy.
Sentinel lymph node biopsies are used to determine whether breast cancer has spread from the primary tumour site into the lymphatic system. The previous standard of care was that surgeons injected a radio isotope near the tumour site which would then migrate to the lymphatic nodes most likely to contain invasive cancer. A gamma probe was then used to locate areas of high radioactive intensity, which provided the surgeon with the location of the lymph nodes for removal and histological analysis. Sentinel lymph node biopsies are a critical step in understanding the stage of breast cancer progression, helping to provide patients with the right treatment, avoiding excessive surgery and minimising post-surgery chemotherapy.

A non-radioactive tracer for cancer staging
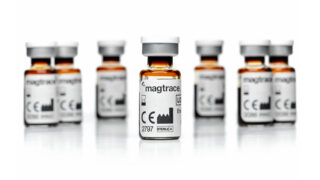
Endomag’s innovative approach was to replace radioactive material with a fluid containing magnetic particles, called the Magtrace® lymphatic tracer. Instead of requiring a gamma probe sensor, it used a highly sensitive magnetic sensing probe, which would then highlight the proximity to the marker lymph nodes on a box called the Sentimag® platform. Proof of concept funding from the UK’s Department of Trade and Industry, followed by seed investment from an investor syndicate led by UCLB, resulted in the development of a prototype solution that was trialled at University College Hospital, London to prove its practical applicability.
Using Magtrace and Sentimag together gave surgeons an option which was procedurally identical to the existing nuclear medicine technique, but with a number of advantages. Dr Eric Mayes, Endomag’s founding CEO explains:
”Unlike the radio isotope, which has a six-hour half-life, Magtrace has a three-year shelf-life and can be injected up to a month in advance of surgery without compromising on signal identification. That effectively removes the supply chain issues, and the need for a nuclear medicine facility, so any clinic can offer a sentinel lymph node biopsy.”
Further clinical studies enabled Endomag to gain CE marks for both Sentimag and Magtrace by 2010. Over the decade that has followed, the company and its strategic distribution partners have developed a highly engaged customer base around the world too, with over 250,000 women in more than 45 countries having been treated using Endomag’s technologies. Recently, Magtrace also received and official guidance recommendation from the National Institute for Care and Excellence (NICE), which is highly influential in validating NHS adoption of technologies.
Importantly, the company’s problem-solving ethos and close relationship with clinicians across the world led to another exciting innovation, a product which helps surgeons to mark the location of the primary breast cancer tumour.
Precise localisation for early-stage tumours
Advances in medical imaging technologies have delivered improvements in breast cancer screening, with tumours now being identified much earlier, often when they are only a few millimetres in size. But when a patient is admitted to hospital for surgery to remove such tumours, that procedure is more challenging for surgeons, as these smaller tumours can be harder to accurately locate. Indeed, until recently the solution was relatively primitive.
As Dr Eric Mayes commented, “For a long time, the standard of care was basically a hook-wire, which was inserted by a radiologist into the site of the tumour, typically on the day of surgery. This had many drawbacks, such as the wire often moving prior to surgery, making it harder to find the target site, and the placement procedure itself causing significant pain and discomfort – sometimes even risk of infection – for the patient.”
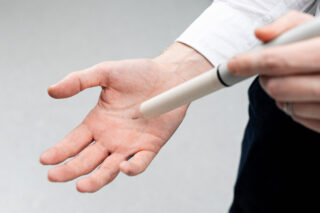
Endomag’s solution was Magseed, a tiny metal seed the size of a grain of rice, which is implanted into the tumour under image guidance. Like Magtrace, it can be located using the Sentimag probe, and removed during surgery. The Magseed’s small size and twisted structure make it unlikely to move, with clinical trials showing that the need for follow-up surgeries is significantly reduced compared to use of a wire. Also, like Magtrace, it enables more efficient use of hospital resources as patients can have Magseed inserted in an outpatient setting and come back days, weeks, or even months later to have surgery. Use of the Magseed was pioneered in some of the world’s leading cancer care centres including London’s Royal Marsden Hospital, and it was featured on the BBC’s prime time show, ‘Trust Me I’m a Doctor’ in 2018.
A long road to success
The road to success certainly involved tackling a plethora of commercialisation challenges. Dr Steven Schooling, who directs the Physical Sciences and Engineering activities at UCLB, worked closely with Endomag’s founders and Eric Mayes to establish and scale the company.
“Creating a successful university spinout company is a complex and time-consuming process as you seek to translate innovative research into solutions that satisfy clear unmet need. Finding the right combination of technology, markets, people, and investors to successfully navigate this pathway is never easy. But, during my 10+ year direct involvement with the Endomag opportunity project, the commitment of the academic founders, Dr Eric Mayes and other members of the senior leadership team that he recruited ensured that challenges such as regulatory hurdles associated with nanoparticles and securing highly motivated distribution partners for the company’s solutions were overcome.”
“With significant year-on-year growth, the upcoming launch of the next generation Sentimag® platform and the development of advanced laparoscopic and endoscopic probes to guide surgeons in melanoma, prostate, thyroid, colon and cervical cancers, it is clear that we are entering a new chapter in the company’s story, with the promise of even greater impact” says Mayes.
“Our mission at Endomag is all about helping clinicians to solve their challenges and provide a better standard of cancer care. We’re proud to be playing a part in developing products that improve outcomes and experiences for cancer patients around the world.”